CAP-1
Monoclonal Antibody(mAb), Anti-CANCER TherapeuticsOverview
-
What is CAP-1?
-
• CAP-1 is a code name as an abbreviation of Cell Aster’s Product (or Project)-1
• CAP-1 is an antibody therapeutics that can overcome drug resistance to existing anti-cancer drugs, and inhibit cancer cell's invasion & metastasis.
• Publications
Type Press Release R&D Paper Title Discovery of key factors in the development of metastatic gastric cancer EPB41L5 Mediates TGFβ-Induced Metastasis of Gastric Cancer Language Korean English • CAP-1 is a new type of drug that aims to inhibit metastasis and invasion, completely different from the mechanism of action of other anticancer drugs currently used, that is, cell growth inhibition and death of primary cancer. It is an anticancer drug with a new concept, which is expected to have excellent effects in preventing invasion and metastasis and to overcome drug resistance to existing anticancer drugs. In other words, it is a global first-in-class (FIC) innovative new drug that works through a new target and mechanism that is completely different from existing drugs, and is highly likely to become a blockbuster drug (annual sales of more than $1 billion).
Backgrounds
Motivations of Research
Poor prognosis & Low survival rate
It is known that metastatic gastric cancer has a very poor prognosis
with a 5-year survival rate of less than 30% worldwide. The incidence of gastric cancer
is high mainly in East Asia, including Korea, Mongolia, Japan, and China.
According to the 2018 cancer incidence and mortality prediction report published in Korea,
the incidence of gastric cancer is the second highest after lung cancer.
However, there are few studies on the corresponding target therapy biomarkers.
The research team selected EPB41L5 as a target due to its high expression in the gastric
cancer patient group with a poor prognosis among the candidate biomarkers for gastric cancer
targeted therapy.
In particular, by confirming that the higher the expression of EPB41L5,
the lower the survival rate of the gastric cancer patient group,
the study was conducted to confirm the possibility
as a new targeted treatment marker.
Limits of treatment for gastric cancer
Even though anticancer chemotherapy is mainly used as a treatment method for gastric cancer
along with a surgical operation to excise cancerous tissue, but it is not accepted as an
internationally standardized treatment.
Although studies are being actively conducted to find biomarkers applicable to targeted therapy
to improve the therapeutic effect of metastatic gastric cancer, the clear molecular cause is not
well known due to the heterogeneity of gastric cancer.
The main cause of gastric cancer metastasis - EMT
The main cause of gastric cancer metastasis is epithelial-mesenchymal transition (EMT).
It has been reported that EPB41L5 mainly contributes to the
development of zebrafish and Drosophila and mouse intestinal
embryogenesis via EMT, but functional studies in cancer are lacking.
Recently, it has been reported that the Arf6/AMAP1/EPB41L5 signaling pathway
contributes to renal cancer and breast cancer metastasis,
but the function and clinical relevance of EPB41L5 in gastric cancer
has not yet been reported.
What We have found
• The research team confirmed that the high expression of EPB41L5 was associated with the low survival rate of gastric cancer patients through oligonucleotide microarray and Kaplan-Meier survival analysis.
• EPB41L5 mRNA and protein expression were increased by TGF-β1 in gastric cancer cell lines, suggesting that EPB41L5 gene expression is regulated by smad-dependent TGF-β signaling.
• Through EPB41L5 gene promoter analysis, a number of smad binding motifs were identified, and by using luciferase reporter gene analysis and chromatin immunoprecipitation (ChIP) analysis, it was found that phosphorylated Smad3 binds to the EPB41L5 promoter –265/-256 site, and affects the transcriptional regulation of EPB41L5.
• EPB41L5 expression increased by TGF-β signaling affected the mobility and invasion of gastric cancer through the epithelial-mesenchymal transition (EMT) process. It was observed that this was significantly inhibited by TGF-β inhibitor, EPB41L5 siRNA and EPB41L5 monoclonal antibody. Moreover, EPB41L5 overexpression promoted gastric cancer metastasis, and it was revealed that p120-catenin, a cell adhesion molecule, is involved in this process.
• The research team observed that gastric cancer metastasis was inhibited by injecting EPB41L5 monoclonal antibodies into gastric cancer metastatic mouse animal models transfected by EPB41L5 overexpression and TGF-β. This suggested the possibility of EPB41L5 as a new gastric cancer target treatment.
Result, Conclusion and Expectation
• It has been revealed that the TGF-β/EPB1L5/p120-catenin axis is a major key molecular mechanism for gastric cancer metastasis.
• Based on the results that high EPB41L5 expression affects the low survival rate of gastric cancer patients, EPB41L5 appears to be a potential diagnostic marker for gastric cancer in the future.
Mechanism Of Action
Over-expression of the EPB41L5 gene by the TGF-β signaling pathway
TGF-β activates EBP41L5 gene expression.
By the TGF-β signaling pathway known to be involved in the growth and metastasis of cancer, Phospho-SMAD binds to the EPB41L5 gene promoter to induce overexpression of the EPB41L5 gene.
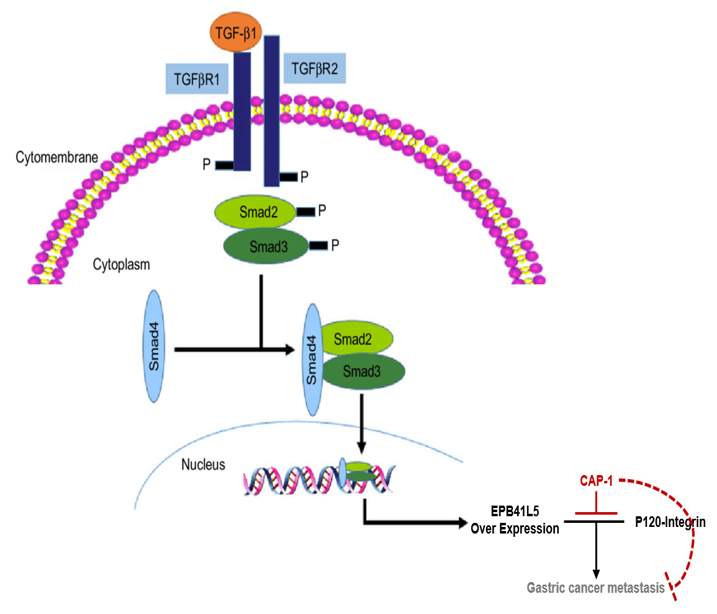
Overexpressed EPB41L5 Protein
Due to the over-expressed EPB41L5 protein, p120-catenin is detached from E-Cadherin, the cell adhesion molecule, and the catenin-actin link centered on E-Cadherin is broken, and EMT, the process of conversion from epithelial to mesenchymal cells, is began and it increases the mobility and invasion of cancer cells.
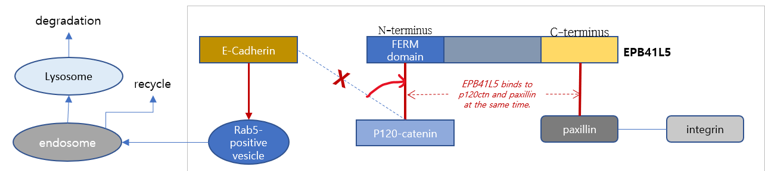
The effects of the targeted therapeutic mAb (monoclonal Antibody), CAP-1
Inhibiting cancer invasion & metastasis, and overcoming resistance to drugs
✔ It inhibits EPB41L5 activity and thereby inhibits EMT by maintaining intercellular binding.
✔ It blocks metastasis of cancer cells by inhibiting the very first step of EMT.
✔ Since EMT has already been identified as a mechanism of resistance to various anticancer drugs, it is expected that drug resistance can be overcome by blocking metastasis.
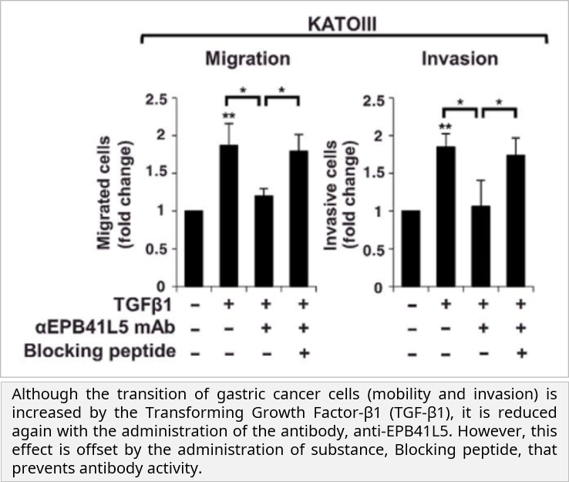
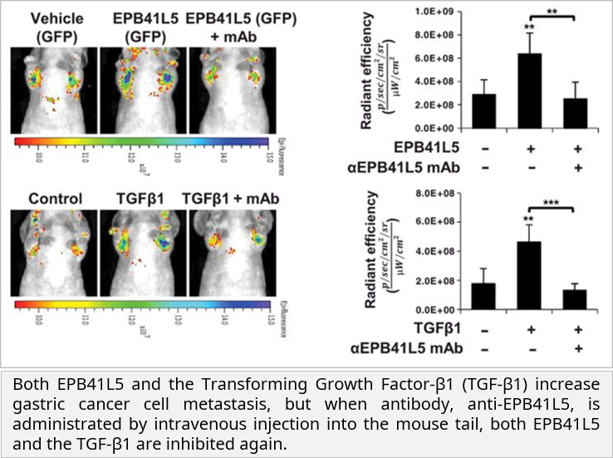
Characteristic
Distinctive points & Therapeutic effects
• It is a promising therapeutic target discovered as a biomarker associated with a poor prognosis through analysis of actual patient clinical data and gene expression data. That is to say, it is a study that starts from clinical observation, not from a molecular biological theoretical hypothesis.
• We have confirmed for the first time in the world that EPB41L5 is a promising therapeutic target for advanced gastric cancer.
• Since the action of EPB41L5 on various cancers (e.g., kidney cancer, non-small cell lung cancer brain metastasis, squamous cell cancer, breast cancer, pancreatic cancer, etc.) continues to be revealed, it is expected to be effective in treating gastric cancer as well as other cancers.
• By blocking the early stages of the cancer metastasis process (EMT), the effectiveness of currently available treatment methods (surgery, radiation, and drugs) can be maximized.
• Since EMT has already been identified as a mechanism of resistance to various anticancer drugs, our treatment is expected to be very effective in overcoming resistance to existing anti-cancer drugs by fundamentally blocking EMT.
• Research on the role of EPB41L5 is still insignificant, but its role will be elucidated through various studies in the future, and consequently EPB41L5 is expected to become a versatile therapeutic target.
• Key researchers who have discovered therapeutic target substances are showing confidence and actively participating in the commercialization process.
• Research is underway to minimize immunogenicity (ADA: Anti-Drug Antibody) by developing fully human antibodies.
Commercialization
Current Status
IP Content
• Ab: Antibody (Paratope)
• Ag: Antigen (Epitope)
• Pc: Pharmaceutical composition
Patents registered in Korea
| IP Reg. Number | IP Content | IP Reg. Date | IP Documents |
|---|---|---|---|
| 10-2125703 | Ab | Jun. 16, 2020 |
Text version (in English / Korean)
PDF version (in Korean) |
| 10-2268846 | Ag | Jun. 18, 2021 |
Text version (in English / Korean)
PDF version (in Korean) |
| 10-2268847 | Pc | Jun. 18, 2021 |
Text version (in English / Korean)
PDF version (in Korean) |
Patents that are pending
| Pub. Country | Pub. Number | IP Content | Pub. Date | IP Documents |
|---|---|---|---|---|
| WIPO (PCT) | WO 2020 / 032614 A1 | Ab, Ag, Pc | Feb. 13, 2020 |
Text version (in English / French / Korean)
PDF version (in Korean) |
| China | CN 112638939 A | Ab, Ag, Pc | Apr. 9, 2021 |
Text version (in English / Chinese)
PDF version (in Chinese) |
| EU | EP 3 835 317 A1 | Ab, Ag, Pc | Jun. 16, 2021 |
Text version (in English / German / Franch)
PDF version (in English) |
| USA | US 2021 / 0252145 A1 | Ab, Ag, Pc | Aug. 19, 2021 |
Text version (in English)
PDF version (in English) |
| Japan | JP 2021 - 533817A | Ab, Ag, Pc | Dec. 9, 2021 |
Text version (in English / Japanese)
PDF version (in Japanese) |
Developing Monoclonal Antibodies(mAbs)
All of following mAbs(monoclonal antibodies) are under efficacy experiments:
• Mouse mAb
• Humanized mAbs
• Fully Human mAbs
Roadmap
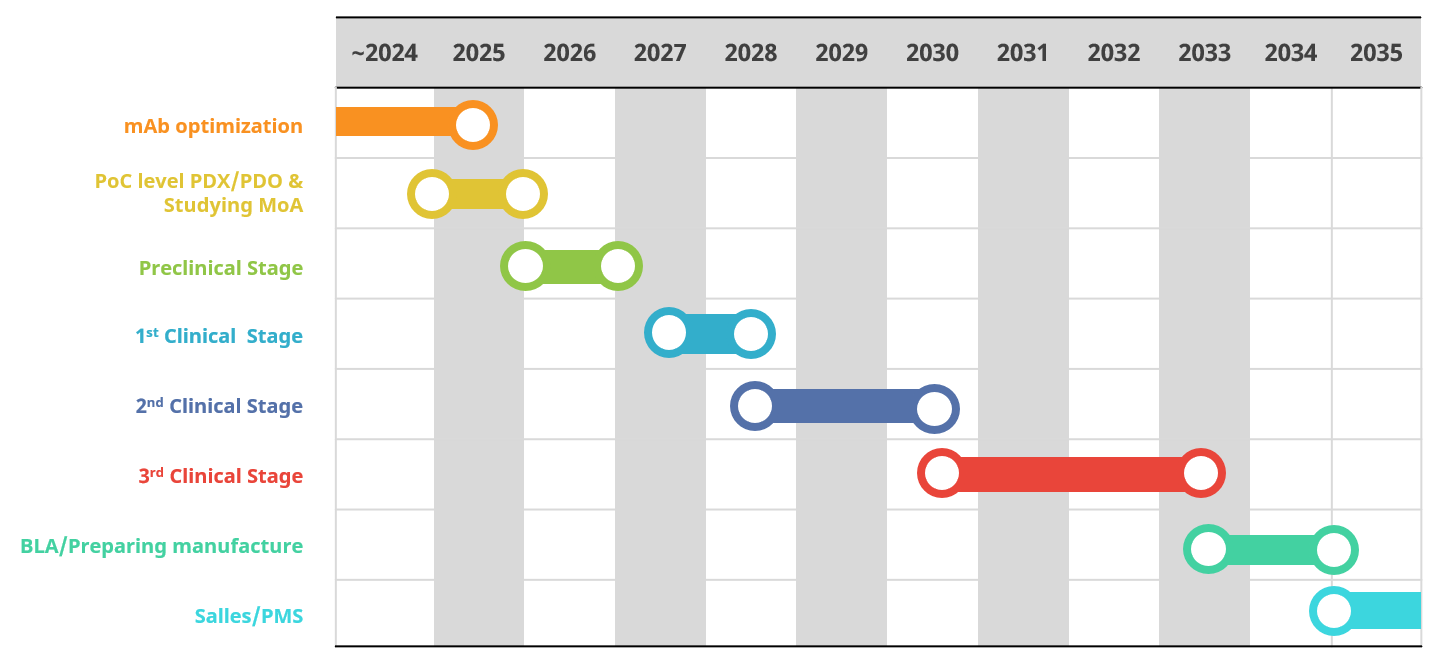
Next Steps
CAP-1 & More...
STEP 1 (CAP-1)
Cancer metastasis prevention function and overcoming existing anticancer drug resistance
Our therapeutic agent that inhibit the biological activity of EPB41L5 are not only expected
to inhibit metastasis(due to invasion & migration), but also to overcome resistance to conventional anticancer
drugs.
The reason is that our therapeutic agent fundamentally block EMT,
which has been identified as a resistive mechanism for various anticancer drugs,
in the early stages.
STEP 2 (CAP-1+)
A new concept anti-cancer treatment
We will develop antibody therapeutics with an added function
to destroy cancer cells (by applying genetic recombinant and/or
antibody engineering technologies such as bispecific antibody,
fusion protein, ADC, etc.).
This drug will be a new concept anti-cancer treatment that blocks
the metastasis of cancer cells and at the same time activates
immune cells to induce the annihilation of cancer cells.
Other Pipelines
We are considering the possibility of developing the following drugs:
• IPF(Idiopathic Pulmonary Fibrosis
• Drug-resistant treatment of cancer cell / tissue
• Treatment for intractable diffuse-type gastric cancer
• NASH(nonalcoholic steatohepatitis) treatment
• PROTACs(Proteolysis Targeting Chimeras)-based treatment
• TRIMAWAY-based treatment
• General CAR-T-based treatment
• Developing Bio-Platform technologies
Clinical Trial
Do you want to try this treatment?
Please wait a moment.
We're doing a series of experiments to confirm the efficacy of mAbs.
We'll contact you as soon as possible, if you leave a message.
